Parasites need to adapt to continue living off their hosts. Almost every type of organism on earth faces parasitism, including us humans. Ecologists have assumed that the parasite has influenced the DNA evolution of its host, and some new science from Israel explores how this relationship works at the gene level.
In the course of evolution, hosts evolve immune response to parasites through genetic change while parasites evolve to overcome host defenses.
In particular, genes of the Major Histocompatibility Complex (MHC), which exist in all vertebrates (that’s us with interior skeletons) are extremely important for an effective immune response because they encode for proteins that recognize foreign parasites such as viruses, bacteria or parasitic worms.
One goal of ‘immunogenetics’, which is the field that explores the relationship between the immune system and genetics, is to understand the evolution of MHC under parasite-mediated selection.
PhD student Shai Pilosof and his colleagues have bridged the disciplines of ‘immunogenetics’ and ‘network ecology’ for the first time. The study just published in Nature Communications demonstrates that the evolution of genes involved in immune response to parasites in one species depends on the whole web of host-parasite interactions in the system.
Until now, the relationship between MHC evolution and parasitism has been explored mainly in systems of one parasite species in populations of a single host species.
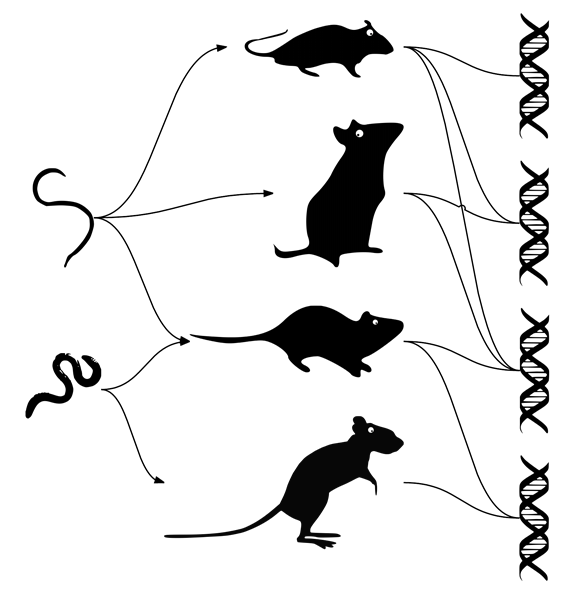
In nature, however, a particular parasite can infect many host species and a host species can be co-infected with many parasite species. This complexity in interactions is crucial because it creates indirect pathways by which infection of one host with a parasite depends on the infection status of other hosts. The best way to depict such complexity is by drawing a network of interactions.
Such networks are extensively studied in the field of ‘network ecology’, which aims to understand the ecological and evolutionary processes underlying the structure of networks of species. Importantly, it is unclear how network structure affects MHC diversity, which in turn is one of the main drivers of host-parasites
interactions.
In the recent study, Pilosof and his colleagues explored this question using data on a community of 14 rodent hosts, their parasitic worms and their alleles of a MHC gene (allele is a molecular variants of a gene), collected in Southeast Asia. A comparison between the structure of a host-parasite network and that of a host-MHC allele network revealed that the structures are highly associated such that hosts infected with similar parasitic worms also harbored similar MHC alleles.
A deeper analysis also discovered groups of MHC alleles and parasites that are more strongly linked to each other (within a group) than with alleles/parasites from other groups in the system. The structure of these groups depended on the structure of the host-parasite network, suggesting MHC-parasite co-evolution at the host community level, rather than at the level of a single species.
The study by Shai Pilosof and his colleagues shows that indirect effects between hosts and parasites affect MHC genetic diversity in more than one species, thereby scaling-up MHC theory from the population level to the community level.
The study also has some applied implications because human-driven environmental changes can alter the interactions between hosts and parasites.
For example, rodents and their associated parasites can invade disturbed areas, possibly creating new interactions between local rodents and invading parasites. Such perturbations to the host–parasite network may have consequences for the evolution of MHC through indirect cascading effects.
Because rodents are carriers of zoonotic parasites (transferred from animals to humans), changes to network structure may have implications for risk of zoonotic diseases. This is where diseases jump between species – diseases like Ebola, avian flu and swine flu as some examples. It is also believed that HIV originated in a primate in Africa so this research has interesting implications for researchers studying disease evolution obviously.
Comments
comments





“… parasitism, including us humans …” Like humankind needed to realize that the Earth revolves around the sun and not vice versa, so we need to realize that ninety (!) percent of “us” is “them”, i.e. by cell count we are only ten percent body cells and 90% “foreign” (which throws some doubts on the “us” and “alien” concept anyhow). By body mass it may be 30/70 bus since not mass, but genes count, we are a minority in our own bodies. That said, it seems logical that gene evolution, whose speed anyhow is not yet fully accounted for, given the time life had to develop on Earth, was largely driven by gene interactions, probably mediated by “vectors” like viruses and we are far more “ancestrally” connected to “lower” life forms than some may feel comfortable with. Yet here may also lie the secrets to combating several as yet intractable diseases.